What Term Do We Use to Describe a Charged Atom
Answer 1 of 6. An atom is electrically neutral - has no overall electrical charge.
What Is A Charged Atom Called Quora
The smallest possible amount of matter which still retains its identity as a chemical element consisting of a nucleus surrounded by electrons.
. An atom a positive charge occurs when an atom has more protons than electrons. The tendency of water molecules to stick together is called. There are 2 places where charge is located in the atom.
The reason is an ion is a charged particle. In an atom with a neutral overall charge the number of protons is equal to the. In DS1 atoms are heated until they are very high energy and unstable.
The nucleus in comparison to the entirety of the atom is the smallest part but holds the most mass. It is denoted with a plus sign. However each atom contains even smaller particles called electrons.
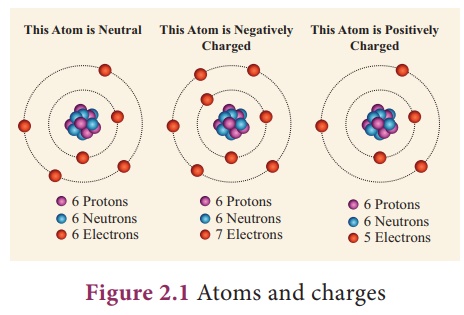
The three parts of the atom are protons positively charged neutrons neutral charge and electrons negatively charged. What is the term for an atom that is electrically charged as a result of gaining or losing electrons. Electric charges may be positive or negative in nature.
Anion negatively charged ionparticle tramwayniceix and 1 more users found this answer helpful. Positively charged subatomic particle forming part of the nucleus of an atom and determining the atomic number of an element. An electrochemical unit of charge the faraday is useful in describing electrolysis reactions such as in metallic electroplating.
However an atom can consist of a single proton ie the protium isotope of hydrogen as. The atom is made of three different parts. Learn vocabulary terms and more with flashcards games and other study tools.
When the electrons hit the atoms in the chamber they cause some of the electrons in the atoms to be. The single most important characteristic of an atom is its atomic number usually denoted by the letter Z which is defined as the number of units of positive charge protons in the nucleus. The atomic mass number is not the same as the atomic mass seen on the periodic table.
A typical atom consists of a nucleus of positively-charged protons and electrically neutral neutrons with negatively-charged electrons orbiting this nucleus. Term used to refer to a charged atom. In the context of chemistry and physics charge usually refers to electric charge which is a conserved property of certain subatomic particles that determines their electromagnetic interaction.
The proton is what determines its positive charge. Nuclear reactions can alter atoms. When an atom.
In ions Electrons Protons - Charge Charge is written with the number before the positive or negative sign Example 1 Note. An atom is the defining structure of an element which cannot be broken by any chemical means. An atom that gains one or more electrons becomes.
A charged atom is called an _____. An atom that loses one or more electrons becomes a positively charged ion called a cation. The Electrons negative charge protons positive charge and the neutrons no charge.
On the other hand if an atom has an unequal number of protons and electrons then the atom is electrically charged and in fact is then referred to as an ion rather than an atom. The nucleus contains neutrons zero charge and protons positive charge Around the nucleus there are electrons located on electron shields and the charge of electrons is equal to the charge of protons in the nucleus but have negative sign. Charged atom charged particle or charged object which sets up the electric field.
A charged atom has either too many or too few electrons. It weighs 1 amu. Term used to refer to a charged atomANSWER.
One faraday equals 96485332123 coulombs the charge of a mole of electrons that is an Avogadros number 602214076 10 23 of electrons. Each electron has a. The charge can be either a positive charge or a negative charge.
The protons and neutrons make up the nucleus the center of the atom and the electrons fly around the nucleus. Charge is a physical property that causes matter to experience a force within an electromagnetic field. To sum it all up we can say that the charge of protons in the.
The are then hit by electrons that are discharged by a cathode ray in the thruster chamber. It attracts negative charges and repels other positive charges. Protons and neutrons form the atomic nucleus.
For example if an atom has a Z of 6 it is carbon while a Z of 92 corresponds to uranium. An atom is a building block of matter that cannot be broken apart using any chemical means. Click here for more information.
What term is defined as an atom that has a charge. Cation positively charged ionparticle Cl-. An atom that has a positive charge is called a cationcat-ion and an atom that has a negative charge is called an anion an-ion.
Any particle whether an atom molecule or ion that contains less electrons than protons is. How are atoms charged.

No comments for "What Term Do We Use to Describe a Charged Atom"
Post a Comment